Kinds of refrigerants
and their effect on the environment
 What are the atmosphere ozone layers What are the atmosphere ozone layers
Under the atmosphere we understand the layers of different gases (N2, О2, СО2 etc.), which are not able to flow into the outer system due to terrestrial gravity. These layers, depending on the height of location, are divided into troposphere, stratosphere, mesosphere and thermosphere. Especially in stratosphere on the height of 15-35 km there are thick layers of О3, which are called "ozone layers".
With the beginning of the 20th century, development of science and technology connected with the growth of industry and life level of people has caused the problem of environmental contamination.
Due to deepening of such problems as depletion of ozone layers, greenhouse effect (increasing of temperature of the earth atmosphere), acid rains, pollution of sea waters, in order to take measures on solving these problems there was accepted the Montreal Protocol dated June 29, 1990, which includes restriction rules of using ozone-depleting substances. Recently in Rio de Janeiro (Brazil) there has been opened the UNO conference on environmental development and there has been taken the decision to investigate concrete offers on the Earth environment protection.
According to the Montreal Protocol, 5 substances of freon family have been admitted the objects for restriction of using ozone-depleting substances: R-11, R-12, R-113, R-114, R-115. Though according to the terms, restriction has been accepted as 50% in 1995, 85% – in 1997, 100% – in 2000, lately the USE, EU and other leading countries have strictly toughened the terms of realization of the Montreal Protocol and put forward the suggestion to reduce the terms of realization since January of 1994 till 85%, and since January of 1996 – complete prohibition of production and using of ozone-depleting substances.
 The principle of formation and distribution of ozone layers The principle of formation and distribution of ozone layers
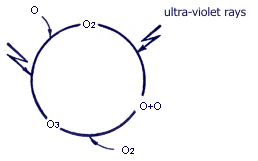 Diatomic oxygen molecule (О2), being underground, under the influence of ultra-violet rays possessing big energy is decomposed to one-atomic oxygen (O). This one-atomic oxygen after connecting again with diatomic oxygen generates ozone (О3). Ozone generated in this way, under the influence of ultra-violet rays, is again re-generated through decomposition and combination. Diatomic oxygen molecule (О2), being underground, under the influence of ultra-violet rays possessing big energy is decomposed to one-atomic oxygen (O). This one-atomic oxygen after connecting again with diatomic oxygen generates ozone (О3). Ozone generated in this way, under the influence of ultra-violet rays, is again re-generated through decomposition and combination.
Though ozone layers are spread from the earth surface up to the height of 60 km, 90% closely abuts to stratosphere (11 - 50 km); at 15-35 km above the earth (thickness of 20 km), especially up to the height of 25 km the biggest thickness is observed (about 100 rrm).
 The role of ozone layers and their depletion The role of ozone layers and their depletion
Absorbing ultra-violet rays radiated by the sun, ozone layers protect the earth from their harmful influence:
- direct harm: skin cancer, cataract, reduction in production of agricultural products, disappearance of the sea plankton, change of the colour and hardness of plastic goods and paints;
- indirect harm: increasing of the sea water level due to melting of ice-house because of the atmosphere temperature increasing ("greenhouse effect").
After 1980, when revealing that separate substances among halogen elements (substances containing F, Cl, Br, I) deplete ozone layers and contribute to the increase of the earth atmosphere temperature, freon containing halogen elements has become the object of restricted use being the ozone-depleting substance.
First, it has been revealed in the middle of 80-ies above the Southern line (during 1977 - 1984 it has been reduced by more than 40%).
Reduction of ozone volume during 1969 - 1986.
(Southern hemisphere: 1.1 – 9%)
(Northen hemisphere: 1.1 – 3.6%)
|



